Professor Hee-Tak Kim, working on a project of KI for the NanoCentury, has developed a lithium sulfur battery (LSB) that realizes 92% of the theoretical capacity and an areal capacity of 4mAh/cm2. LSBs are gaining a great deal of attention as an alternative for lithium ion batteries (LIBs) because they have a theoretical energy density up to six to seven times higher than that of LIBs, and can be manufactured in a more cost-effective way. However, LSBs face the obstacle of having a capacity reaching its theoretical maximum because they are prone to uncontrolled growth of lithium sulfide on the electrodes, which leads to blocking electron transfer.
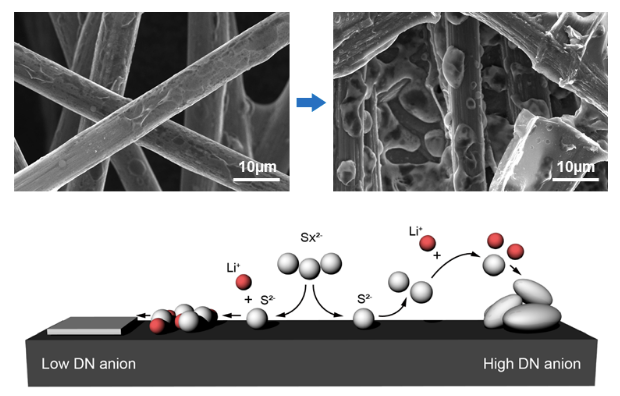
To address the problem of electrode passivation, researchers introduced an additional conductive agent into the electrode; however, it drastically lowered the energy density of LSBs, making it difficult to exceed 70% of the theoretical capacity. Professor Hee-Tak Kim and his team replaced the lithium salt anions used in conventional LSB electrolytes with anions with a high donor number. The team successfully induced the three-dimensional growth of lithium sulfide on electrode surfaces and efficiently delayed the electrode passivation.
Based on this electrolyte design, the research team achieved 92% of the theoretical capacity with their high-capacity sulfur electrode (4mAh/cm2); this capacity is equivalent to that of a conventional LIB cathode. Furthermore, the team was able to form a stable passivation film on the surface of the lithium anode, which exhibited stable operation over 100 cycles. This technology, which can be flexibly used with various types of sulfur electrodes, marks a new milestone in the battery industry.
This work was published in Nature Communications in Jan. 2019 and selected as an ‘Editor’s highlight’.
Prof. Hee-Tak Kim Department of Chemical and Biomolecular Engineering, KAIST
Homepage: http://eed.kaist.ac.kr
E-mail: Heetak.kim@kaist.ac.kr






