The electrochemical CO2 reduction reaction (CO2RR) has received considerable attention because, using renewable energy sources such as solar energy, it can convert CO2 into chemical feedstock or even useful fuels. However, the electrochemical CO2RR in aqueous solutions often encounters several challenges including poor energy efficiency and carbonaceous product selectivity. The poor energy conversion efficiency originates from the high overpotential required for converting stable CO2 into other chemical species. Also, the similar redox potential between the hydrogen evolution reaction and the CO2RR results in the co-production of hydrogen, which causes low selectivity of the CO2 reduction products.
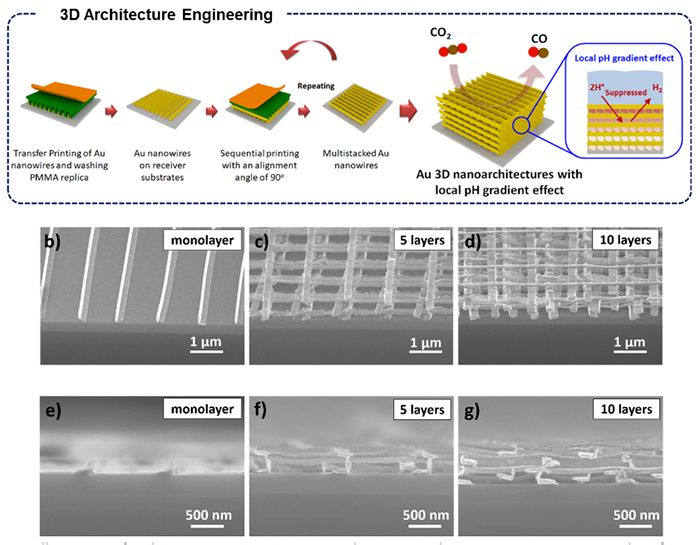
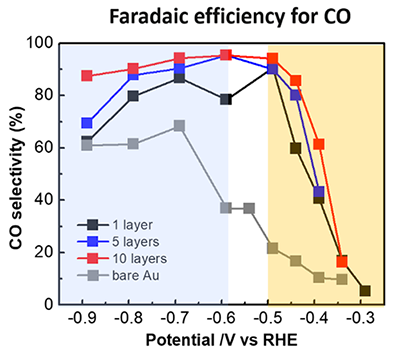
Based on their unique solvent-assisted nanotransfer printing (S-nTP) technology, combined with oblique-angle evaporative deposition, the joint research team lead by Prof. Jihun Oh and Prof. Yeon Sik Jung in the Department of Materials Science and Engineering at KAIST developed a multi-stacked 3-dimensional Au catalyst architecture. They were able to control the grain orientation and effectively increase the fraction of so-called “high-index grains” to maximize CO selectivity over the hydrogen evolution reaction. They achieved CO selectivity of over 90% in a wide range of applied cathodic potentials; this value is substantially higher than the 22 % of flat Au thin films. Finally, they demonstrated versatile transferability of the 3D catalyst onto a gas diffusion electrode in a flow reactor, maximizing CO evolution with an exceptional mass activity of ~172.66 A g-1 (highest among reported values so far) at an overpotential of only 80 mV. Prof. Oh said “Well-defined nano and microstructured catalysts will provide profound insight into design selective and efficient CO2RR catalysts.”
Prof. Yeon Sik Jung, Prof. Jihun Oh Dept. of Materials Science and Engineering, KAIST
Homepage: http://funnano.kaist.ac.kr
E-mail: ysjung@kaist.ac.kr






