An important aspect of the future for rechargeable batteries lies in the development of advanced electrode materials that not only possess reliable electrochemical performances capabilities but also safe, sustainable, and cost-effective. In this respect, organic molecules with redox-active motifs are witnessing a surge in popularity as attractive candidates for next-generation electrodes. The research groups of Prof. Seokwoo Jeon, Prof. Paul V. Braun, and Prof. Il-Doo Kim have focused on revolutionizing rechargeable organic batteries through the structural engineering of organic-based electrodes. They succeeded in developing a new 3D periodic nano-network of multi-redox active polyimide with incomparably high-rate cycling stability. The study was published on August 6, 2021, in Energy & Environmental Science (Impact Factor: 38.5).
Redox-active organic materials offer the inherent advantages of lightness, a high theoretical capacity, a small environmental footprint, and molecular tunability. While there has been significant progress in the exploration of a new class of redox centers and molecular structures, the practical requirements of high electrochemical activity and stability alond with rapid kinetics for fast charging, are motivating the search for rational design principles applicable to electrode architectures.
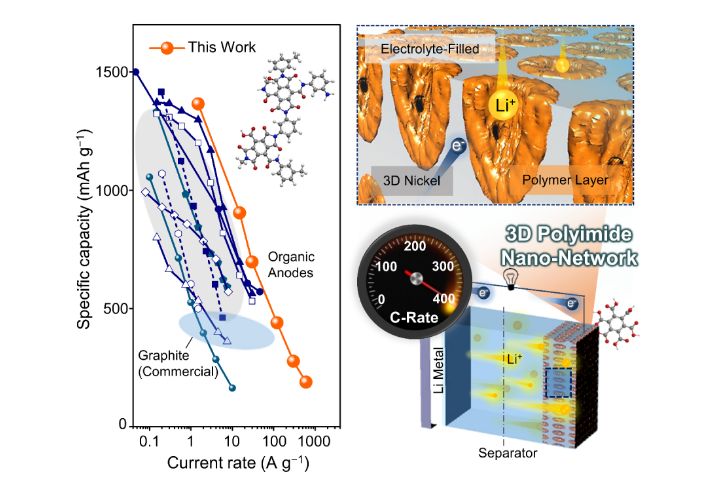
The researchers conceived a lithographic fabrication strategy to realize a 3D bicontinuous nano-network consisting of a periodically porous nickel-supported redox-active organic layer (pore radius <300 nm). The engineered 3D nano-network electrode enabled fast electrochemical reaction kinetics by providing efficient pathways for electrons and ions within the electrode, as well as pathways for rapid ion transport within the continuous, electrolyte-filled porous network. On the basis of the foregoing principal benefits, they observed promoted ‘super-lithiation’ phenomenon in nearly all unsaturated C=C bonds in multi-carbonyl polyimide, realizing a high reversible capacity (1260 mAh g−1) and 82.8% capacity retention over 250 cycles at a 10C rate. The strength of the proposed concept was further corroborated by the unprecedented high-rate capability (achieved rates up to 400C). The researchers elucidated the origin of the ultra-high -rate capability through a kinetic analysis of the superior charge transfer accompanied by an explanation of the redox reaction chemistry. ultimately, the full anode-basis capacity (2810 mAh cm−3) was shown to surpass the target line required to meet the energy targets (1500 Wh L−1 on the half-cell level) for fast-charge hybrid electric vehicles, suggesting its strong potential as a future alternative to the traditional inorganic anodes.
“The architecture of the 3D bicontinuous nano-network in this study holds great significance as a general platform for organic electrodes.” Prof. Jeon said. “We believe that we have taken an important step toward eco-friendly and promising energy storage.”
This research was supported by the Creative Materials Discovery Program through the National Research Foundation of Korea. This work was also supported by the Office of Naval Research through the Defense University Research-to-Adoption Initiative.
Youngjin Ham, Prof. Seokwoo Jeon, Prof. Il-Doo Kim Dept. of Materials Science and Engineering, KAIST
Homepage: http://fdml.kaist.ac.kr
E-mail: jeon39@kaist.ac.kr






