Organ-selective drug delivery holds the potential to effectively treat organ-associated diseases while minimizing systemic side effects and to enable the use of modalities whose utility is currently limited by delivery issues. Although lipid nanoparticle- and polymersome-based organ-selective delivery systems have recently been reported, their delivery has been limited to organs of the mononuclear phagocyte system (MPS), such as the liver, spleen, and lungs. Moreover, since surface-formed protein coronas mediate organ targeting in these systems, concerns may arise regarding the risk of unexpected organ tropism due to variations in plasma protein patterns between patients. Therefore, a new delivery platform is needed to enable tissue- and cell-selective delivery of therapeutics to diverse organs beyond those of the MPS.
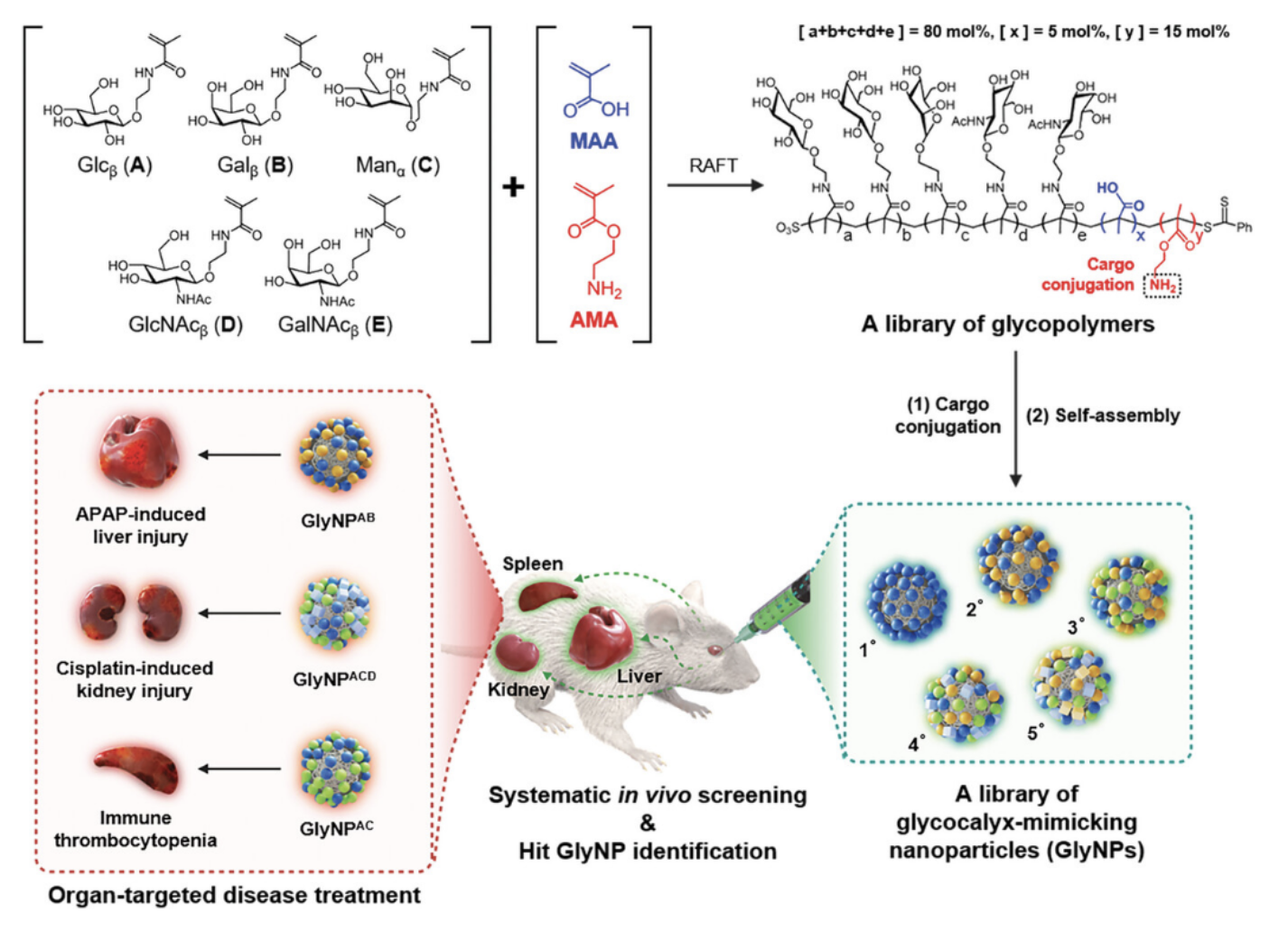
A joint research team, consisting of Professor Sangyong Jon’s group from the Department of Biological Sciences at KAIST and Professor Hee-Seung Lee’s group from the Department of Chemistry at KAIST, has developed a library of glycocalyx-mimicking nanoparticles (GlyNPs) that mimic the pericellular carbohydrate coat, which is ubiquitously present on cells and plays a crucial role in numerous biological processes, such as cell–cell recognition, cell–extracellular matrix interactions, and cell adhesion. The study was published in Advanced Materials on March 15, 2024 (Glycocalyx-mimicking nanoparticles for organ-selective drug delivery and therapy, Advanced Materials, 2311283 (2024)) and was selected as a cover paper.
A library of 31 GlyNPs was constructed through random copolymerization of five key sugar moieties prominently found in naturally-occurring carbohydrates. Using this library, GlyNP hits with differential selectivity for liver, spleen, lung, kidneys, heart, and brain were identified by systematic in vivo biodistribution screening and were subsequently confirmed to show cellular tropism in the target organ. Additionally, loading of each liver-, kidney-, and spleen-selective GlyNP hits with the anti-inflammatory molecule or a representative drug yielded therapeutic efficacy in various organ-associated disease models, demonstrating that the new GlyNP platform enables organ- and cell-targeted delivery of therapeutic cargoes.
“In our new GlyNP library platform, organ-selective delivery does not rely on surface-formed protein coronas but rather on specific sugar interactions, and is not limited to the MPS organs. We envision that our organ-selective delivery platform can be expanded to therapeutics whose use has so far been limited by delivery issues across various patient populations,” said Sangyong Jon, the corresponding author and a professor in the Department of Biological Sciences and the KAIST Institute for the Biocentury at KAIST.
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF2018R1A3B1052661 and 2018R1A5A1025208), funded by the Ministry of Science and ICT.
Prof. Sangyong Jon, Dohyeon Kim Dept. of Biological Sciences
E-mail: syjon@kaist.ac.kr
Homepage: http://www.bionanolab.co.kr






