Professor Ji-Ho Park’s group at KAIST has developed an innovative cancer treatment, engineering mRNA-loaded lipid nanoparticles (LNPs) to enhance immune cell activation directly within solid tumors. This approach addresses the limitations of conventional cell-based immunotherapies—which often involve complex, time-consuming, and costly ex vivo cell preparation processes—while still demonstrating robust therapeutic efficacy. Compared to traditional cell therapies, mRNA-based treatments offer improved accessibility for patients and hold promise for broad applicability across a wide range of solid tumors, including colorectal cancer and skin cancer.
The team’s approach focused on activating and expanding tumor-infiltrating lymphocytes (TILs) directly in the tumor microenvironment. TILs have previously shown strong anti-tumor potential through the cytotoxic activity of CD8+ T cells when administered as an adoptive cell therapy. To induce potent, localized TIL activation, the team used LNPs to deliver mRNA encoding a membrane-anchored anti-CD3 single-chain variable fragment (MA-aCD3), engineering both macrophages and cancer cells in tumors after administration. Macrophages are another type of immune cell present in tumors and they can naturally support TIL function though costimulatory and cytokine signaling. However, the engineered expression of MA-aCD3 on their membranes enables them to directly activate TILs, strengthening their antitumor functions and inducing proliferation. Additionally, the expression of MA-aCD3 on cancer cells mimics the action of bispecific T cell engagers (BiTEs), another popular cancer therapy, facilitating direct interaction between TILs and tumor cells and promoting targeted cytotoxicity.
In preclinical models of solid tumors, intratumoral administration of the mRNA-LNPs resulted in strong activation and expansion of TILs, particularly CD8+ T cells, and thereby effectively suppressed tumor growth and extended survival without any observable adverse effects. The researchers also explored the therapeutic synergy between their mRNA therapy and anti-PD-1 checkpoint blockade—a widely used immunotherapy that has limited efficacy in certain resistant melanoma models. Strikingly, the combination therapy exhibited enhanced antitumor effects.
This work presents a significant step forward in the development of next-generation cancer treatments, offering a highly adaptable strategy for in situ immune modulation in solid tumors.
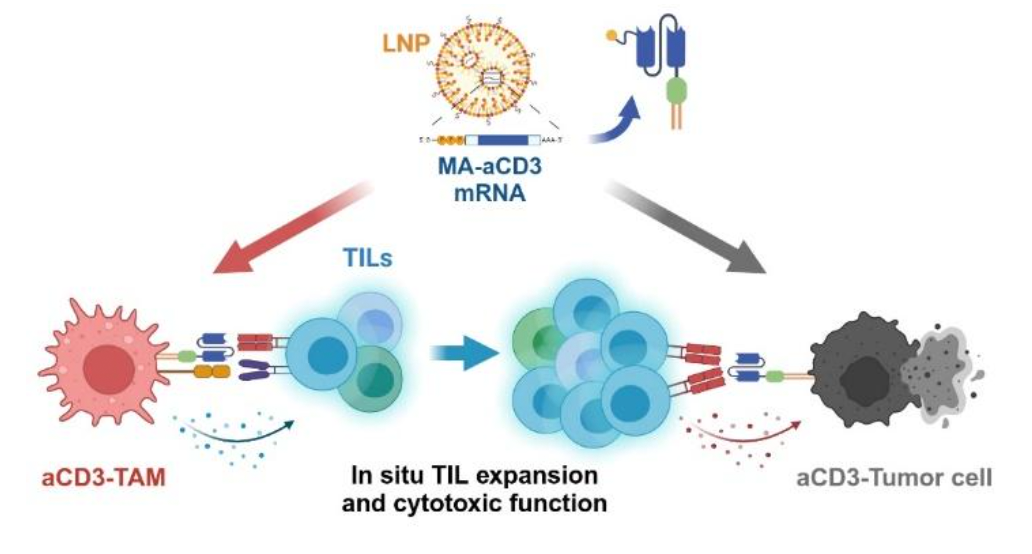
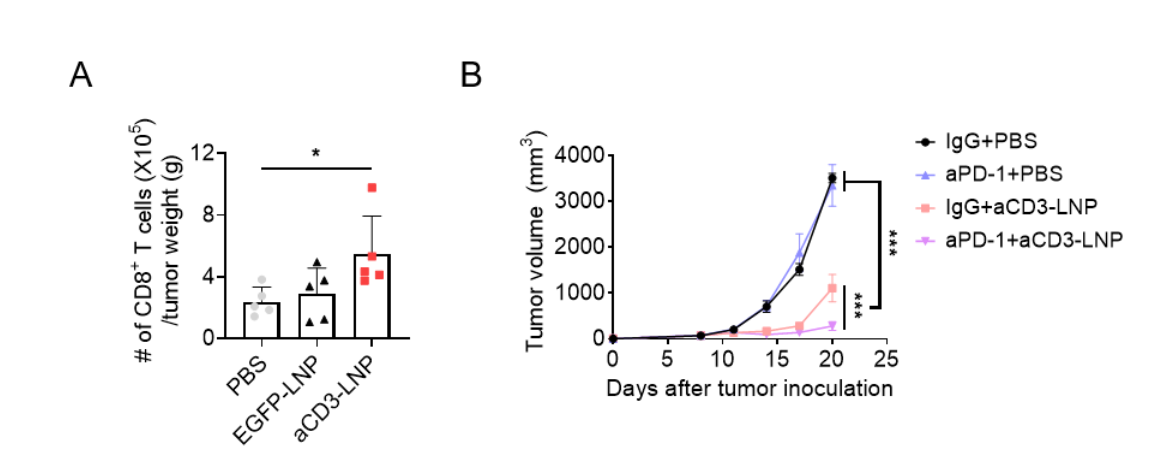
This work was supported by grant NRF-2021R1A2C2094074 of the Basic Science Research Program of the National Research Foundation funded by the Ministry of Science and Information and Communication Technologies (ICT), Republic of Korea.
Junyong Yoon, Erinn Fagan, Moonkyoung Jeong, Ji-Ho Park Department of Bio and Brain Engineering, KAIST
E-mail: jihopark@kaist.ac.kr
Homepage: https://openwetware.org/wiki/Park_Lab






