The precise inhibition of target proteins can serve as an effective means to dissect complex signaling networks or identify new therapeutic targets. Existing techniques include the widely-used genetic approaches; however, not all proteins can be studied in this way because some proteins are so critical to an organism’s development that gene knockout in early development results in death. To address these drawbacks, small-molecule drugs have been developed but they do not allow researchers to study highly-localized effects and are poorly reversible.
The Korean research group, led by Professor Won-Do Heo in the Department of Biological Sciences at the Korea Advanced Institute of Science and Technology (KAIST) reported the development of a new technology that overcomes these challenges named Light-Activated Reversible Inhibition by Assembled Trap (LARIAT).
The LARIAT system consists of a blue-light-mediated interaction of plant Cryptochrome 2 (CRY2) with CIB protein and a multimeric protein (MP). The light-sensitive protein CRY2 forms clusters with the protein CIB1 linked to a multimeric protein in the presence of blue light. These clusters serve as synthetic intracellular compartments to conditionally trap and, thereby, inactivate target proteins fused to CRY2 upon blue-light illumination. This method provides researchers with a reversible and tunable way to control protein functions.
In addition, by linking CRY2 with a single-domain antibody against the green fluorescent protein (GFP), LARIAT expanded the application to control the function of any protein tagged with GFP.
Utilizing this system, researchers demonstrated the ability to effectively and reversibly inactivate diverse biological processes, including cell morphology, cell migration, and cell division with high-spatiotemporal resolution by using LARIAT.
This new technology can provide an unprecedented opportunity for investigating diverse protein functions in the dynamic intracellular environment.
The research team published the LARIAT technique in the June edition of Nature Methods.
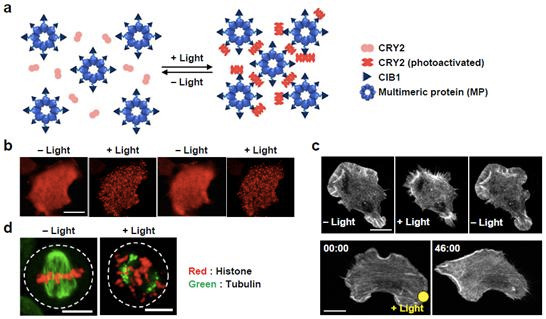
b. Reversible cluster formation upon blue-light stimulation.
c. Spatiotemporal control of cell morphology and cell migration using LARIAT.
d. Temporal control of cell division by LARIAT.