Professor Yong-Hoon Kim’s laboratory at the Graduate school of Energy, Environment, Water and Sustainability (EEWS) has revealed the electrochemical reaction mechanism of edge-selenated graphene nanoplatelets for dye sensitized solar cells (DSSCs). This research provides valuable theoretical guidelines for the realization of high-performance DSSCs in the future through computer simulations.
Carbon-based materials have a great potential to replace precious metal catalysts because of its abundance in nature, durability in electrochemical environments, and good electrocatalytic activity that can be induced by edge or surface functionalization. As promising alternatives to metal electrocatalysts, carbon-based materials have been actively studied for the counter electrode (CE) in DSSC, which typically consist of a transparent conducting oxide, TiO2 coated with a dye as the active electrode (photoanode), an electrolyte, and a CE. The key roles of CE in DSSCs are to transfer electrons from the external circuit to the electrolyte and to catalyze the reduction of the redox couple. As a CE material, platinum (Pt) performs the best but it is a high-cost precious metal that is limited in supply. Carbon-based materials are considered to be a promising alternative, but they have been only active enough in one type of commonly used (cobalt-based) electrolytes. For the iodide/triiodide (I-/I3-) electrolytes that are more common and desirable for efficient reaction kinetics, the performance of carbon-based electrocatalysts has not been satisfactory. Recently, an experimental group led by Professor Jong-Beom Baek at UNIST synthesized edge-selenated graphene nanoplatelets (Se-GnPs) and found that their performance as a CE material with I-/I3– is remarkably comparable to that of Pt. The atomistic origin of the excellent performance of Se-GnPs with I-/I3– was shown by Professor Kim’s team at EEWS, KAIST via ab initio atomistic simulations. Together, the research teams published this work in Science Advances in June 2016 (DOI: 1.1126/sciadv.1501459).
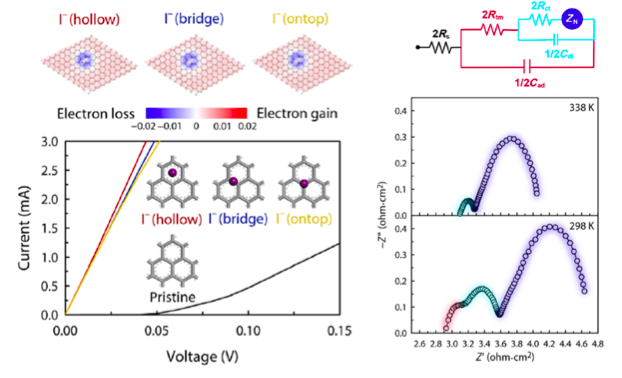
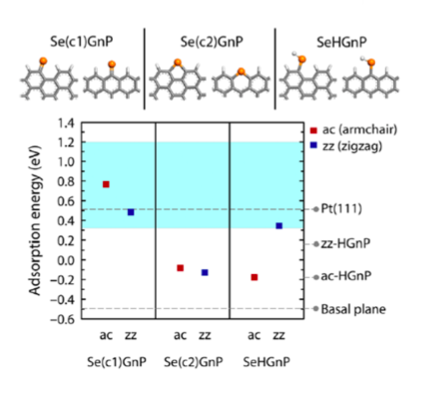
First-principles or ab initio simulation is a powerful theoretical/computational tool that can provide atomic-scale information on various material properties that are often difficult to obtain within experiments. For example, the mechanism of the excellent DSSC CE performance of the newly synthesized Se-GnP was experimentally unknown, making materials optimization and further development difficult. Carrying out ab initio atomistic simulations, KAIST team was able to explain the mechanism of electrochemical reaction involving Se-GnPs with I-/I3-. Specifically, they identified that single-coordinated C=Se bonds established along both armchair and zigzag GnP edges (Figure 1, top left panel) result in high electrocatalytic performance and structural stability in electrochemical environments.
In addition, they successfully explained the origin of an anomalous first semicircle that appears in the electrochemical impedance spectra, which is common to carbon-based CEs but has not been understood so far (Figure 2). They showed that I- and I3- induce an n-type doping of graphene or make graphene electron-rich, thus improving the graphene conductivity significantly.
Combining the two results, it is clear that the excellent DSSC CE performance results from two factors. First, single-coordinated Se doping sites along the GnP edges initiate the outstanding triiodide reduction reaction (iodine reduction reaction) process. Thus, well-defined and high-density Se doping of GnP edges is the key for the experimental achievement. Next, as long as the hexagonal sp2 carbon network of graphene is well preserved, the iodine reduction reaction occurring at the Se-GnP edges can be efficiently sustained by the electrons that have entered from the external circuit and rapidly traveled through the electron-rich graphene basal plane. Namely, preserving the pristine graphene basal plane during the synthesis of nanoplatelets is another important requirement (Main image).
The successful theory-experiment collaboration between the KAIST and UNIST groups demonstrates the significant potential of graphene as a catalyst and should open up new opportunities in future solar cell research and applications.
Kim, Yong-Hoon (Associate Professor, Graduate School of EEWS)
Homepage: http://nanocore.kaist.ac.kr/yhklab
E-mail: y.h.kim@kaist.ac.kr