The dilemma of graphene coating: To be or not to be hydrophobic
The hydrophobicity (or philicity) of a surface tells us how the surface interacts with water. When the surface of an object is composed of hydrophilic material (typically polar substances such as metal oxides), a water drop spreads on the surface making a small (<90°) contact angle. Conversely, water drops maintain their shape on a hydrophobic surface to minimize unfavorable interactions. In addition to chemical composition, surface topology profoundly affects hydrophobicity of a surface. Microscopically non-flat surfaces contain air pockets (which are very hydrophobic) and often behave in a superhydrophobic manner (a contact angle > 150°) where the air pockets support water drops avoiding contact with the actual surface. There are certain advantages when the surface is hydrophobic; for example in a water harvesting application a hydrophobic surface lets condensed water drops roll much more easily, thus increasing the efficiency of collecting water from the air.
Graphene, a single atomic-layer sheet of carbon atoms arranged in a hexagonal lattice, has unique properties such as outstanding mechanical strength, transparency, high flexibility, conductivity, high impermeability to gas, as well as thermal and chemical stabilities. It is possible to transfer a graphene layer to a surface (usually via a wet chemical transfer technique) to form graphene-coated surfaces, which inherit the superior properties of graphene and become anti-corrosive and resistant to chemical oxidation. However, due to the atomic thickness of a graphene layer, the hydrophobicity of graphene-coated surface is not solely dictated by graphene but also affected by the underlying surface.
The contact angle of graphene is ~100° meaning just slightly hydrophobic by itself. Graphene becomes “wetting-transparent” when coated onto a hydrophilic surface and allows for interaction between the underlying surface and water through the graphene layer. However, graphene becomes “wetting-opaque” and governs the hydrophobicity of the surface when the underlying surface is more hydrophobic than graphene. This implies that a graphene-coated surface cannot be more hydrophobic than graphene. This applies to microtextured surfaces as well, since conventional graphene transfer techniques let the flat graphene layer “sit” on the surface therefore shading the surface texture beneath.
Conformal graphene coating by CVD
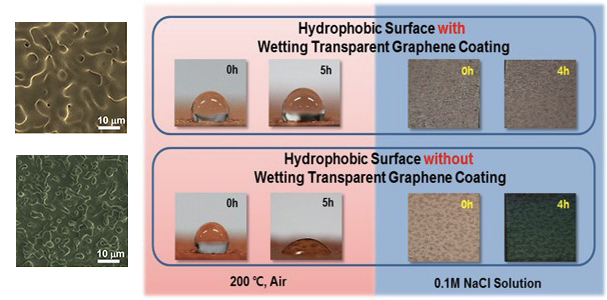
Researchers at KAIST, led by Professor Il-Kwon Oh in the Division of Ocean System Engineering and Graphene Research Center at the KAIST Institute, tackled this problem by developing a new graphene-coating technique. In collaboration with Professor Nikhil Koratkar, at the Rensselaer Polytechnic Institute, they found that graphene grown by thermal chemical vapor deposition (CVD) on a roughened copper surface (contact angle was ~128°) could conform perfectly to roughness features with good adhesion. In contrast to the previous results using transferred graphenes, this in-situ graphene-coating technique preserves the surface texture and therefore maintains strong water repellency (showing contact angle of ~125°), meaning that the graphene-coated surface is “wetting-transparent” even though the underlying surface is more hydrophobic than graphene. The team recently published these striking results in Advanced Materials. The paper was highlighted on the inside front cover of the coming issue on August 13.
In order to create the appropriate surface texture, the team electroplated copper oxide particles onto copper foil which resulted in a hierarchically roughened surface. Thermal annealing at 850°C reduced the copper oxide into copper and subsequent CVD, using methane as the hydrocarbon source, promoted adsorption of carbon atoms on the surface and the formation of a graphene monolayer following the surface topology.
This graphene coating method was also highly resistant to corrosion. No oxidized copper species were detected when the graphene-coated copper sample was heated to 200°C in the air for four hours, and the contact angle was maintained after dipping the sample in 0.1 M NaCl aqueous solution for five hours. “It means our graphene coating really protects the copper surface and maintains the surface topology,” said Gun-Tae Kim, a graduate student in the Division of Ocean System Engineering, who is the first author of the paper.
Promise in water harvesting
There are many places that require such coating with excellent anti-corroive and wetting-stability effects. Components in a desalination plant, such as heat exchangers and condensers, could be protected from oxidation and corrosion caused by seawater. Another application could be water harvesting via dehumidification, which the team demonstrated in the paper. 30% more water could be collected with the graphene-coated copper surface compared to a flat-copper surface without graphene coating.
“On the hydrophobic graphene-coated surface, water condenses to form drops and falls down rapidly,” said Professor Oh. Importantly, the graphene-coated surface maintains its water-harvesting capability while a non-coated copper surface will gradually lose it as surface oxidation will increase the interfacial thermal resistance and hinder heat transfer, which is critical in condensation. The research team even speculated that it may be possible to achieve superhydrophobic graphene-coated surfaces by controlling the surface roughness, which will open more applications in surface science.
This work was supported by the Ministry of Trade, Industry and Energy through the Technology Innovation Program (Graphene Materials and Components Development Program of MOTIE/KEIT 10044410) and a National Research Foundation of Korea Grant funded by the Korean Government (2012R1A2A2A01047543).