Professor Cafer T. Yavuz’s research team is studying highly efficient catalysts for dry reforming of methane to resolve both environmental and industrial problems. Carbon dioxide (CO2) in the atmosphere is steadily increasing and leading to serious environmental problems, particularly climate change. As CO2 accounts for approximately 75% of all greenhouse gas emissions, scientists have focused on reduction of CO2 by capturing and converting it into more useful chemicals. One of the most viable reactions is dry reforming of methane, in which methane and CO2 react to produce hydrogen and carbon monoxide, so-called syngas. The syngas is then used to make plastics and other important petrochemicals.
Catalysts play a pivotal role in dry reforming due to harsh reaction conditions. In general, dry reforming requires at least 800 o C to maximize syngas yield, at which the catalyst is deactivated by sintering and coke formation. Noble metal catalysts, such as Pd, Pt, Ru, Rh, and Ir, have been extensively studied for dry reforming catalysts, but their cost is a major obstacle to commercialization and industrial applications. As an alternative, nickel has gained tremendous attention due to its high activity and affordable price.
Our findings in the Saudi Aramco–KAIST CO2 Management Center project demonstrate that magnesium-oxide-supported nickel-molybdenum catalyst (NiMoCat) has exceptional activity and durability toward dry reforming of methane without deactivation. An important development is the use of affordable nickel nanocatalysts that are prepared in a special manner and show outstanding activity and stability for 600 hours under reactive conditions. The catalysts can also be synthesized by commercial chemicals up to 10 g, which is a crucial factor in industrial applications. We confirmed that such results could not be achieved by conventional methods.
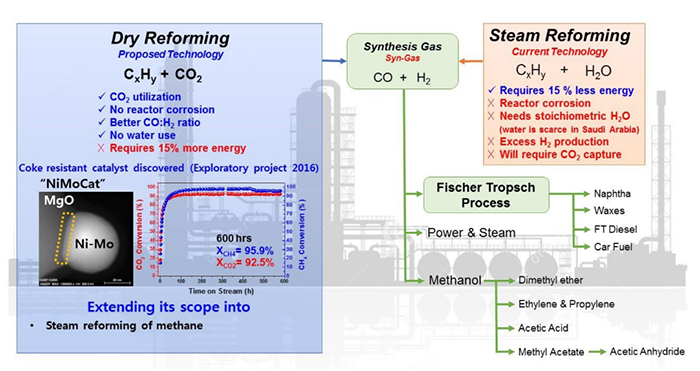
To further optimize and extend the scope of this research, the atomic-level of the reaction mechanism needs to be studied. Therefore, we are continuing to study the key concepts of the activity. Once fully explained, the catalysts will be applied to all reforming reactions (particularly steam reforming of methane) with suitable modifications to establish a new industrial standard and a highly profitable business that is based on CO2 utilization for the Saudi Aramco–KAIST joint venture.
To accomplish our objectives, we will optimize of reaction parameters for all reforming reactions. In doing so, a library of catalysts will be synthesized with variation of catalyst components. The catalysts will be exhaustively characterized through texture analysis and surface science. Their activity and durability will be tested with online GC/BID and MS, and th morphology of the NiMoCat will be controlled through pelletization processes. Finally, pilot- scale testing will be conduced after large-scale synthesis of the NiMoCat is achieved.
The successful completion of this project will provide tremendous financial and strategic advantages to both KAIST and Saudi Aramco as the leading companies and institutions are continuously looking for ways to sequester CO2 for application in useful and profitable products.
Cafer T. Yavuz (Graduate school of EEWS)
Homepage: http://yavuz.kaist.ac.kr
E-mail: yavuz@kaist.ac.kr






