Supported by Saudi Aramco – KAIST CO2 management center, Professor Hyunjoo Lee’s research team developed light-assisted CO2 hydrogenation by investigating metal-CO2 interaction upon light irradiation.
Metal nanoparticles loaded onto supports have been investigated as heterogeneous catalysts for thermal CO2 hydrogenation. When light is irradiated on the metal catalyst, hot electrons can be generated, helping the surface reaction. Inter-band transitions, which occurs by ultraviolet light, are typically observed on precious metals. The input energy is quite high in this case, and photo-excitation by visible light would be more desired. Recently, it was reported that adsorbates on the metal surface can also absorb light, generating hot electrons. If the electrons on the adsorbates can be excited by visible light, the surface reaction would be accelerated by shining light with weak energy.
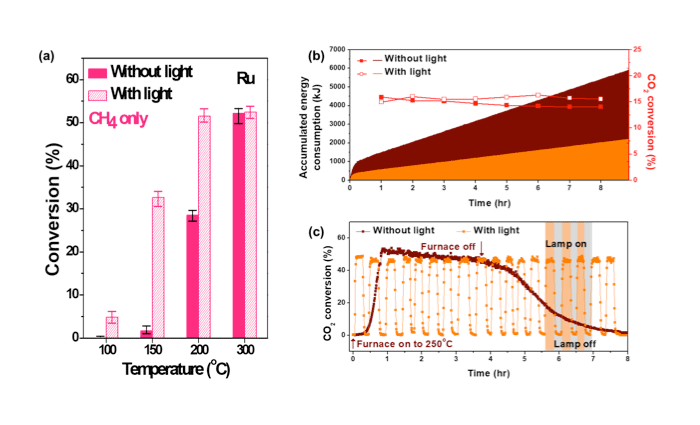
Herein, CO2 hydrogenation on Ru nanoparticles was greatly promoted by light irradiation. The DFT calculation showed that the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) decreased from 8.5 eV in free CO2 to 2.4 eV in CO2 adsorbed on the Ru surface. By investigating the dependency on light intensity and light wavelength, it was confirmed that hot electrons were generated upon light irradiation, enhancing the surface reaction. Very weak light energy such as a laser pointer (532 nm) could enhance the surface reaction. As a result, the light-assisted CO2 hydrogenation reduced energy consumption by 63%, comparing to the case without light irradiation. Additionally, the reaction can be easily turned on and off by turning on or off the lamp. This unique property can be potentially used to produce methane fuel from CO2 and H2 on-site using minimized energy.
This study entitled “Energy-Efficient CO2 Hydrogenation with Fast Response Using Photoexcitation of CO2 Adsorbed on Metal Catalysts” was published in the journal ‘Nature Communication’ on August 2, 2018.
Prof. Hyunjoo Lee (Department of Chemical and Biomolecular Engineering)
Homepage: http://catmat.kaist.ac.kr
E-mail: azhyun@kaist.ac.kr






