A collaborative research team led by Professors Gwangrog Lee, Won-Ki Cho, and Seyun Kim has uncovered key mechanisms governing gene expression in mammalian cells.
Inositol phosphates synthesized by enzymes involved in inositol metabolism serve as essential signaling messengers in eukaryotic cell signaling. These metabolites play critical roles in various physiological processes and are linked to diseases such as cancer, obesity, diabetes, and neurological disorders. The team demonstrated that inositol polyphosphate multikinase (IPMK), a key enzyme in this metabolic pathway, functions as a crucial transcriptional activator within core gene expression networks in mammalian cells.
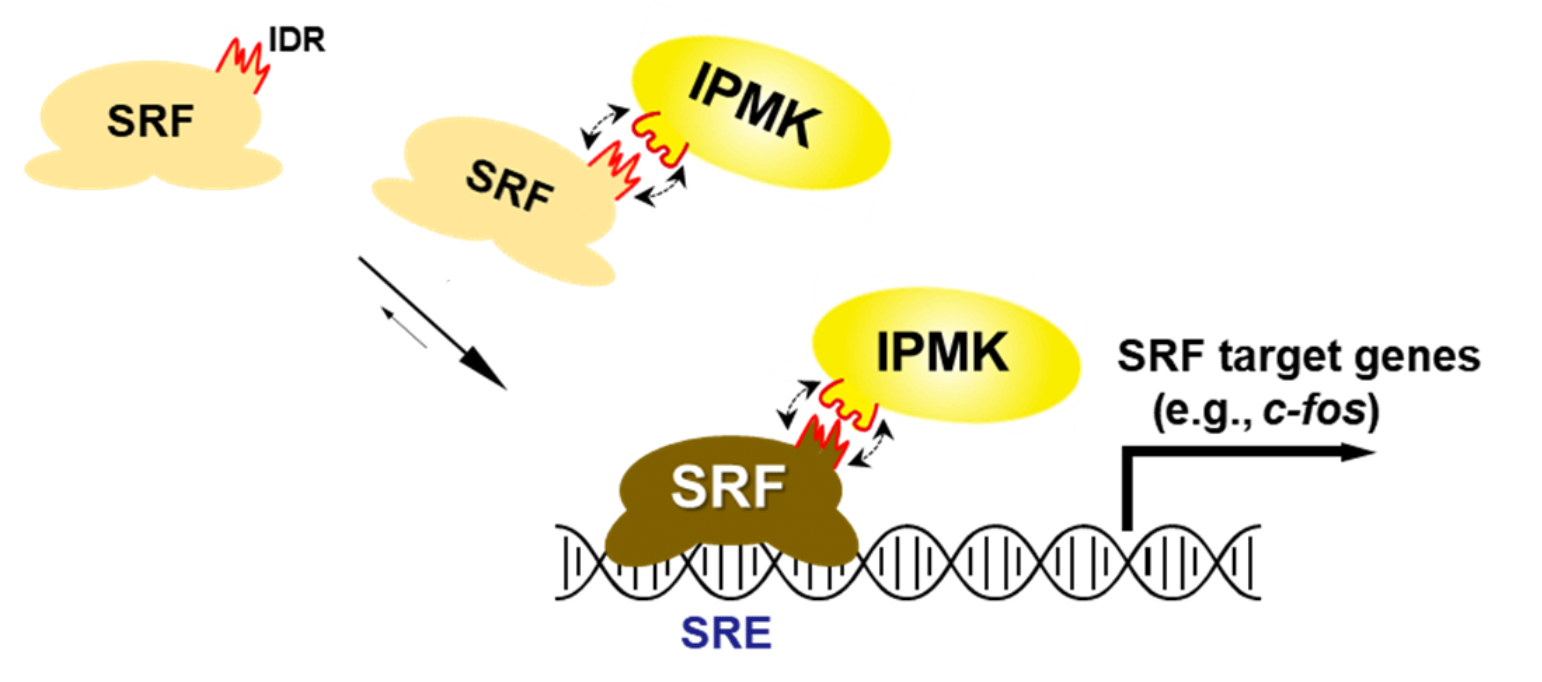
Serum response factor (SRF), a key transcription factor, directly turns on the expression of 200–300 genes involved in cell growth, proliferation, apoptosis, and motility, and is essential for organ development, particularly in the heart. The researchers discovered that IPMK binds directly to SRF, inducing structural changes within the protein. This protein-protein interaction indeed enhances SRF’s transcriptional activity, thereby acting as a regulatory switch that amplifies its function.
Further investigation revealed that disrupting the IPMK-SRF interaction significantly reduced SRF’s activity, leading to severely impaired gene expression. The study also highlighted the role of SRF’s intrinsically disordered region (IDR) and emphasizes the broader biological significance of intrinsically disordered proteins (IDPs). Unlike most proteins that adopt well-defined structures, IDPs remain structurally flexible yet perform essential biological functions, attracting emerging scientific interest.
Professor Seyun Kim remarked, “This study establishes IPMK as a key transcriptional coactivator within the core gene expression network of mammalian cells. We hope this discovery will pave the way for innovative therapeutic strategies, particularly for diseases linked to dysregulated SRF activation such as cancer metastasis.”
The findings were published on January 7 in Nucleic Acids Research (IF=16.7, top 1.8% in Biochemistry and Molecular Biology) under the title “Single-molecule analysis reveals that IPMK enhances the DNA-binding activity of the transcription factor SRF”.
This research was supported by the National Research Foundation of Korea’s Mid-career Research Program, Science Research Center Program, and Basic Research Laboratory Program, as well as by the Suh Kyungbae Science Foundation and the Samsung Future Technology Development Program.
Prof. Seyun Kim Dept. of Biological Sciences, KAIST
E-mail: seyunkim@kaist.ac.kr
Homepage: https://sites.google.com/site/seyunkimlab/






