A research team at KAIST, led by Distinguished Professor Sang Yup Lee, has achieved a significant breakthrough in microbial production of succinic acid (SA), a key platform chemical used for the synthesis of bioplastics, food additives, and pharmaceuticals. By optimizing the magnesium transport system in Mannheimia succiniciproducens, the researchers developed a microbial strain capable of producing SA at the highest productivity ever reported, marking a major step toward sustainable biomanufacturing. This study was published in the September issue of the Proceedings of the National Academy of Sciences (PNAS).
As global environmental challenges such as climate change intensify, the demand for eco-friendly alternatives to fossil fuel–based chemical production continues to rise. Among bio-based platform chemicals, succinic acid stands out due to its wide range of industrial applications. However, achieving industrially competitive microbial production of SA has remained a longstanding challenge. Moving beyond traditional chemical synthesis methods, the research team addressed this challenge by employing systems metabolic engineering.
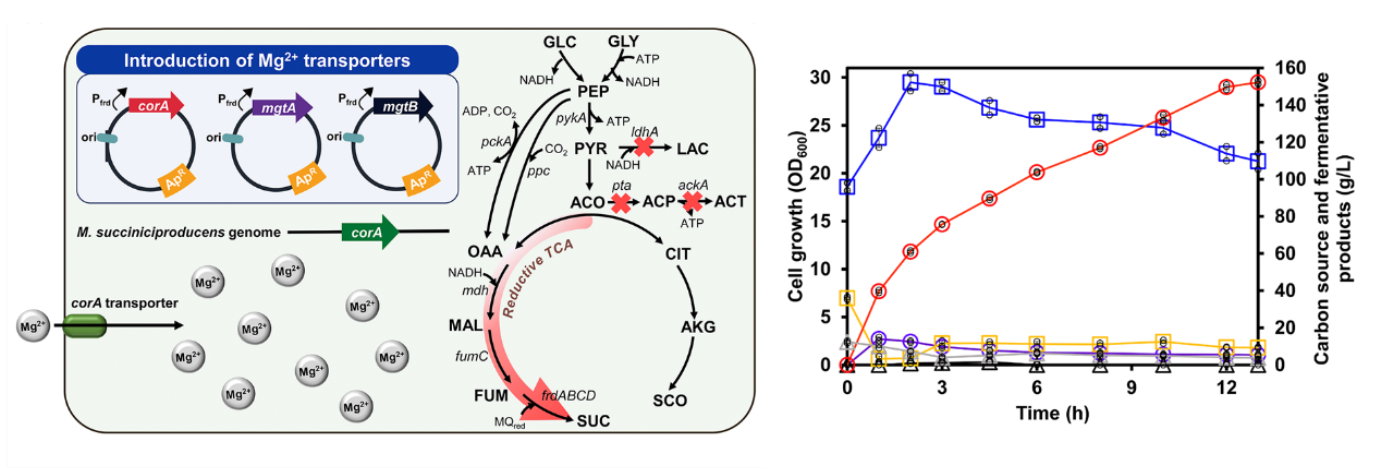
The team focused on the magnesium transport system of M. succiniciproducens, a microorganism originally isolated from the rumen of a Korean cow. During fed-batch fermentation, alkaline neutralizers such as magnesium hydroxide (Mg(OH)2) are used to maintain pH. Through a detailed physiological analysis, the team discovered that magnesium plays a vital role in both cell growth and SA production. They identified corA as the native magnesium transporter gene and further enhanced Mg2+ uptake by introducing magnesium transporters from other microorganisms. Notably, overexpression of mgtB gene from Salmonella enterica significantly improved SA production. The final strain achieved a record-breaking SA titer of 152.23 g/L and a maximum productivity of 39.64 g/L/h, nearly twice the previously reported highest value.
This achievement sets a new benchmark for bio-based SA production and underscores the potential of metabolic engineering in enabling biorefineries to replace petrochemical processes. “Optimizing the magnesium transport system enabled us to produce SA at remarkably high concentrations. This approach offers a promising strategy for developing microbial cell factories to produce other valuable bio-based chemicals,” said Ji Yeon Kim, a co-first author.
Professor Lee also emphasized the broader implications of the work: “Our research not only sets a new standard for SA production but also enhances the economic feasibility of bio-based chemicals, contributing to the development of a sustainable biochemical industry.”
This work was supported by the projects titled development of next-generation biorefinery platform technologies for leading bio-based chemicals industry (2022M3J5A1056072) and development of platform technologies of microbial cell factories for the next-generation biorefineries (2022M3J5A1056117) through the National Research Foundation supported by the Korean Ministry of Science and ICT.
Prof. Sang Yup Lee, Ji Yeon Kim, Dr. Jong An Lee, Dr. Jung Ho Ahn
Dept. of Chemical and Biomolecular Engineering, KAIST
E-mail: leesy@kaist.ac.kr
Homepage: https://mbel.kaist.ac.kr






