The therapeutic synergy of reducing both plaque cholesterol and macrophages through Cargo-switching nanoparticles (CSPN) was demonstrated for prevention of atherogenesis and regression of established plaques (Affinity-Driven Design of Cargo-Switching Nanoparticles to Leverage a Cholesterol-Rich Microenvironment for Atherosclerosis Therapy, ACS Nano, 14, 6, 6519-6531 (2020)).
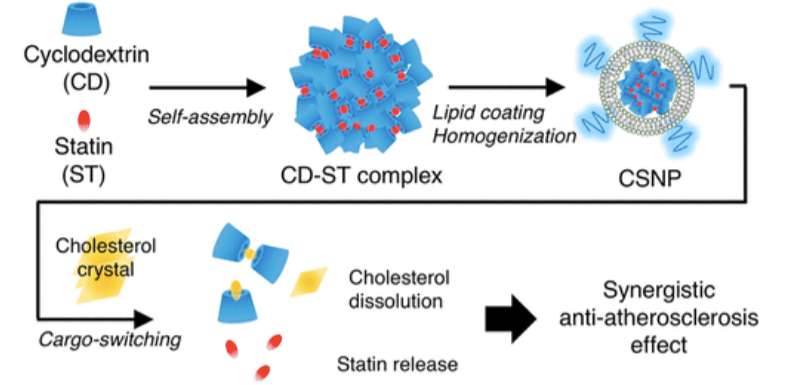
Atherosclerotic plaques exhibit high deposition of cholesterol and macrophages. These are not only the main components of the plaques but also key inflammation-triggering sources. However, no existing therapeutics can achieve effective removal of both components within the plaques. To address the issue, Dr. Ji-Ho Park’s group designed CSNP, which are physicochemically designed to bind to cholesterol and release an anti-inflammatory drug in the plaque microenvironment. CSNP have a core–shell structure with a core composed of an inclusion complex of methyl-β-cyclodextrin (cyclodextrin) and simvastatin (statin), and a shell of phospholipids. Upon interaction with cholesterol, which has higher affinity to cyclodextrin than statin, CSNP release statin and scavenge cholesterol instead through cargo-switching. CSNP exhibit cholesterol-sensitive multifaceted antiatherogenic functions attributed to statin release and cholesterol depletion in vitro. In mouse models of atherosclerosis, systemically injected CSNP target atherosclerotic plaques and reduce plaque content of cholesterol and macrophages, which synergistically leads to effective prevention of atherogenesis and regression of established plaques.
Therapeutic nanomedicine can be engineered to exploit various microenvironmental factors of a disease such as pH, temperature, reduction/oxidation status, and enzymes to improve disease-specificity and therapeutic efficacy. Thus far, no drug delivery system has been developed to exploit a cholesterol-rich microenvironment for effective treatment of a disease. Furthermore, while these disease-associated abnormal microenvironmental factors were exploited simply to stimulate the function of the accumulated nanomedicine, few attempts have been made to normalize the abnormal microenvironment simultaneously. CSNP were physicochemically designed to release low-affinity hydrophobic drug in the cholesterol-rich atherosclerotic microenvironment and scavenge high-affinity cholesterol within the microenvironment. The inherent capacity of CSNP to scavenge and deplete plaque cholesterol in the process of drug release led to superior antiatherogenic efficacy in mouse models of atherosclerosis compared to conventional drug delivery system using liposomes. Although it has been reported that local delivery of statin resolves inflammation and reduces plaque content, the affinity-driven design of CSNP enabled not only local delivery of statin but also depletion of cholesterol within plaques, which significantly improved atherosclerosis management. These findings suggest that CSNP can provide a therapeutic platform for interfacing with cholesterol-associated inflammatory diseases such as atherosclerosis.
Dr. Heegon Kim, Prof. Ji-Ho Park Dept. of Bio and Brain Engineering, KAIST
Homepage: http://openwetware.org/wiki/Park_Lab
E-mail: jihopark@kaist.ac.kr






