Prof. Won Do Heo’s group at KAIST has developed an optogenetic technology called Opto-vTrap, a light-inducible and quickly reversible inhibition system to temporarily trap transmitter-containing vesicles from vesicular exocytosis. Opto-vTrap can be used widely in cells, brain slices, and animal behavioral experiments with fast recovery and without affecting membrane potential. The study was published on 2 February 2022 (Opto-vTrap, an optogenetic trap for reversible inhibition of vesicular release, synaptic transmission, and behavior. Neuron. Volume 110, Issue 3. Pages 423-435.e4 (2022)).
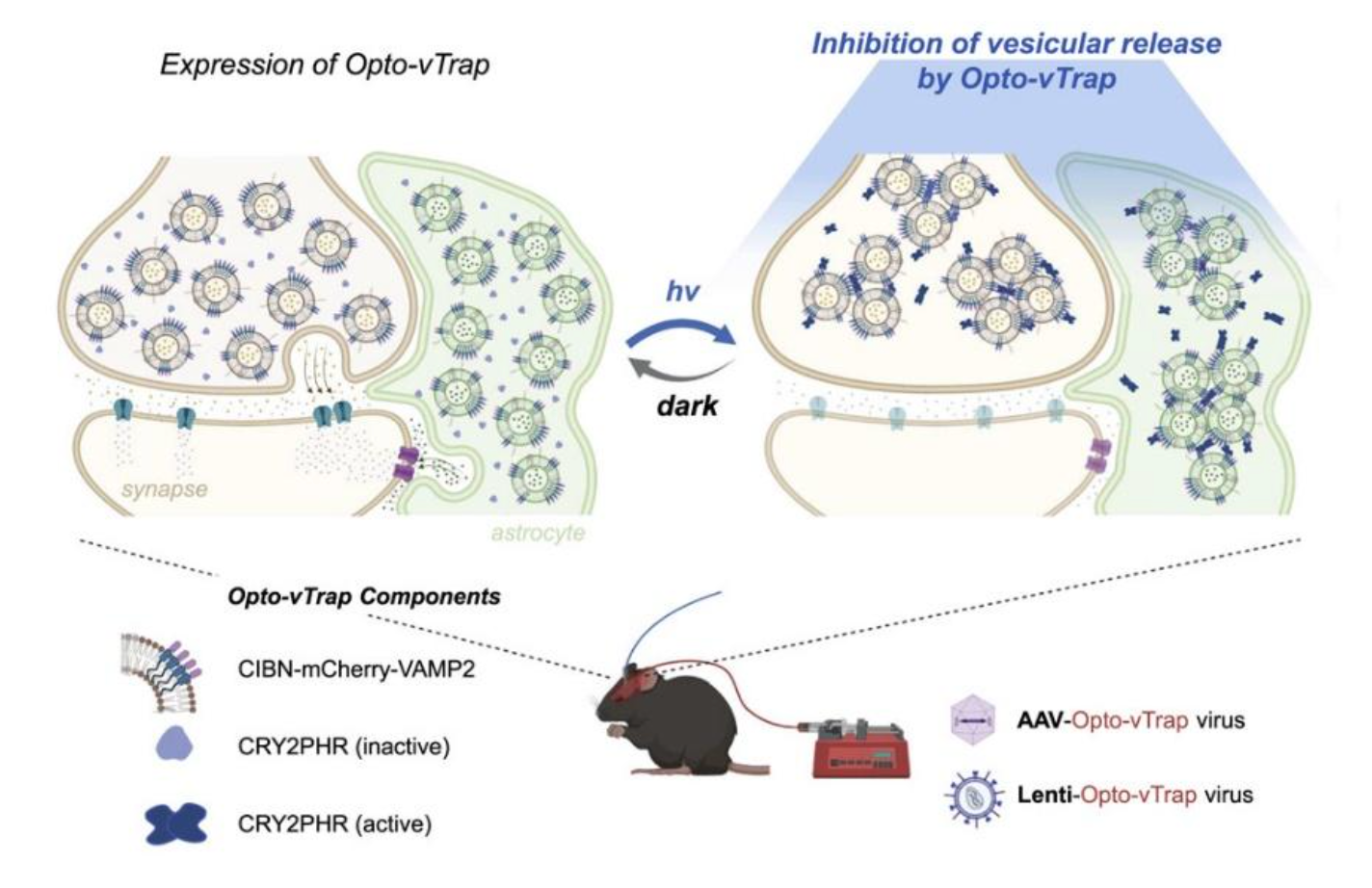
Controlling signal transmission and reception within brain circuits is necessary for neuroscientists to achieve a better understanding of the brain’s functions. Communication among neuron and glial cells is mediated by various neurotransmitters released from the vesicles through exocytosis. Thus, regulating vesicular exocytosis can be a possible strategy to control and understand brain circuits. However, it has been difficult to freely control the activity of brain cells in a spatiotemporal manner using pre-existing techniques. One is an indirect approach that involves artificially controlling the membrane potential of cells, but it comes with problems of changing the acidity of the surrounding environment or causing unwanted misfiring of neurons. Moreover, it is not applicable for use in cells that do not respond to the membrane potential changes, such as glial cells. To address this problem, professor HEO Won Do at Korea Advanced Institute of Science and Technology (KAIST) developed Opto-vTrap, a light-inducible and reversible inhibition system that can temporarily trap vesicles from being released from brain cells. Opto-vTrap directly targets transmitters containing vesicles; it can be used in various types of brain cells, even ones that do not respond to membrane potential changes.
To directly control the exocytotic vesicles, the research team applied a technology they previously developed in 2014, called light-activated reversible inhibition by assembled trap (LARIAT, Nature Methods. 2014). This platform can inactivate various types of proteins when illuminated under blue light by instantly trapping the target proteins, like a lariat. Opto-vTrap was developed by applying this LARIAT platform to vesicle exocytosis. When the Opto-vTrap expressing cells or tissues are shined under blue light, the vesicles form clusters and become trapped within the cells, inhibiting the release of transmitters. Most importantly, the inhibition triggered using this new technique is temporary, which is very important for neuroscience research. Opto-vTrap directly controls the signal transmitters’ release, enabling the researchers to freely control brain activity. The research team verified the usability of Opto-vTrap in cultured cells and brain tissue slices. Furthermore, they tested the technique in live mice, temporarily remove fear memory from fear-conditioned animals.
In the future, Opto-vTrap will be used to uncover complex interactions between multiple parts of the brain. It will be a highly useful tool for studying how certain brain cell types affect brain function in different circumstances. Professor Heo stated, “Since Opto-vTrap can be used in various cell types, it is expected to be helpful in various fields of brain science research,” He explained, “We plan to conduct a study to figure out the spatiotemporal brain functions in various brain cell types in a specific environment using Opto-vTrap technology.”
This work was supported by the KAIST Institute for the BioCentury; and the National Research Foundation of Korea (NRF-2020R1A2C3014742). This paper is based on research conducted as part of the KAIST-funded Global Singularity Research Program for 2021.
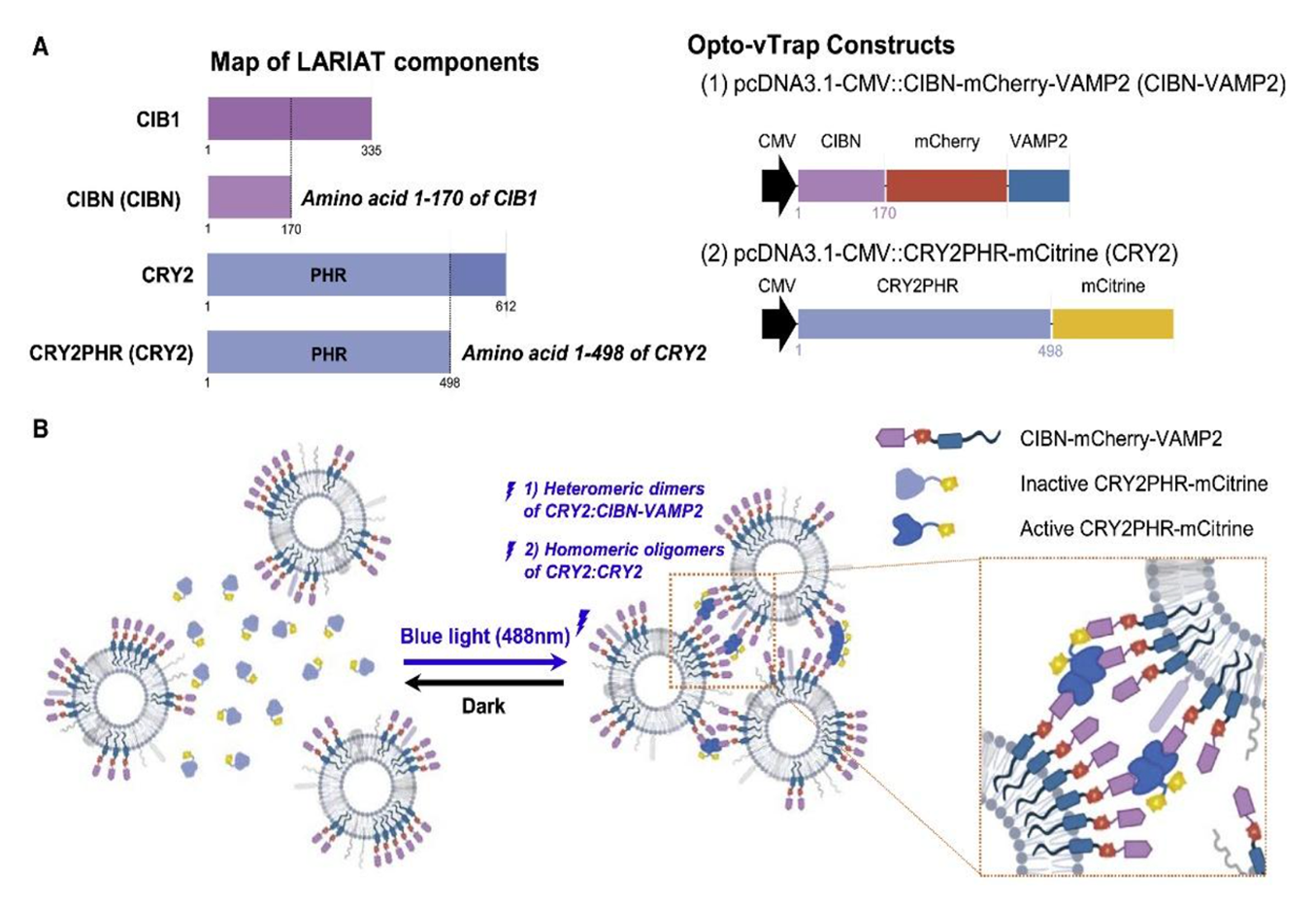
(A) Map of LARIAT components and the Opto-vTrap constructs.
(B) Experimental scheme of working principle of Opto-vTrap. Blue light (488 nm) illumination triggers heteromeric dimerization of CRY2:CIBN-VAMP and homomeric oligomerization of CRY2:CRY2 and induces vesicle clusterization. Thus, CIBN-VAMP2 targeting vesicles are reversibly clusterized upon blue light illumination.
Prof. Won Do Heo, Dr. Joungha Won Dept. of Biological Sciences, KAIST
Homepage: https://sites.google.com/view/heolab/home
E-mail: wondo@kaist.ac.kr






