Lung cancer diagnosis in the future: sniffing one’s breath
Early diagnosis is very important for the effective treatment and improvement of the survival rates of patients, which is considered especially critical for lung cancer. Statistics show that a recent study from the National Lung Cancer Screening Trial states that lung cancer mortality could be reduced by as much as 20% through the benefits of early detection. The survival rate of lung cancer patients increased from 3.5% to 85% when early diagnosis occurred according to the New England Journal of Medicine in 2011.
Lung cancer is usually detected by computer tomography (CT) and expectoration tests in hospitals. Though very powerful and accurate, the current techniques suffer from high cost of operation and difficulty of sampling. As more and more people are being exposed to the threat of lung cancer, demand for new ways to diagnose lung cancer has increased. A popular question has become, “Could we purchase lung cancer diagnostic kits at pharmacies and test for it at home?”
Many researchers believe that breath analysis can be a promising tool for this goal. Breath analysis offers information about a person’s medical conditions through analyzing specific volatile organic compounds (VOCs) in exhaled breath. There are differences in the composition of VOCs in the exhaled breath of lung cancer patients and healthy people. VOCs such as toluene, isoprene, methanol, acetone, and propanal are produced significantly more by lung cancer tumor cells, increasing their concentration (tens to hundreds ppm) in the exhaled breath of lung cancer patients. Therefore, it will be possible to detect lung tumors in people if there is a way to sniff their breath and tell the difference. This would be an ideal way to diagnose lung cancer as breath sampling is not painful (imagine that otherwise you are going to fill a syringe with your blood) and can be performed many times without any drawbacks.
Molybdenum disulfide chemiresistor as a gas sensor
Gas chromatographies in labs could do the breath analysis precisely but it is still not possible to make a one-time use gas chromatography kit that can be sold in pharmacies. Chemiresistor, a material that changes its resistance upon exposure to chemical substances such as VOCs, is a promising candidate as it can be integrated into a portable and inexpensive detection kit. As the resistance change varies by the chemicals, monitoring a current across a channel made of the chemiresistor material can, in principle, tell which gas is flowing through the channel. For accurate diagnoses, especially at the early stages of cancer, it is essential to develop a high-performance chemiresistor which can accurately recognize the target VOCs.
In this manner, molybdenum disulfide (MoS2), a two-dimensional semiconducting nanomaterial with a large surface area has certain advantages. When VOCs are adsorbed on the surface of MoS2, the electrical properties are greatly altered resulting in high sensitivity as a chemiresistor. However, the possibility has not been fully realized yet due to the lack of practical and large-scale fabrication methods of MoS2. Professor Hee-Tae Jung’s group at KAIST developed a new method for fabricating a MoS2-based sensing device. They reported this method in Nano Letters in September of 2014.
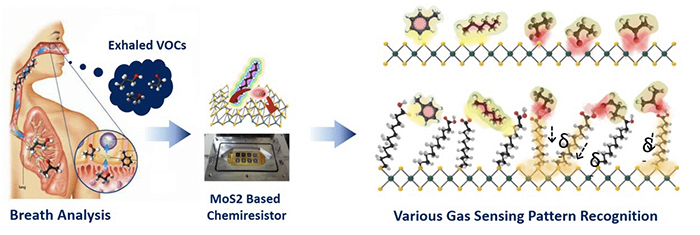
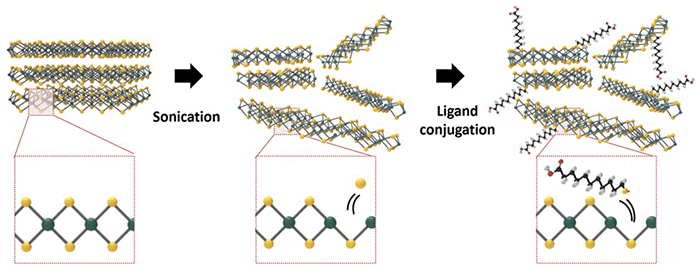
In order to fabricate MoS2-containing chemiresistors, the research team developed a method to generate MoS2 films from a MoS2 dispersion. They ground and sonicated MoS2 powder to form a MoS2 dispersion in an ethanol/water mixture, and then produced the MoS2 film through vacuum filtering of the dispersion. They could further functionalize the MoS2 surface in the dispersion state by conjugating it with a thiolated ligand with a long alkyl chain and a pendent carboxylic acid (11-mercaptoundecanoic acid) utilizing the S defect generated during the sonication step.
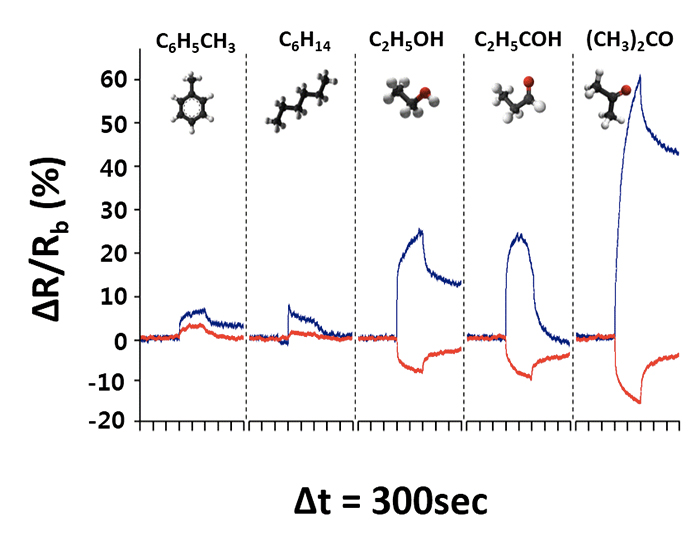
Both pristine and thiolated MoS2 sensing channels exhibited high sensitivity to representative VOCs at a concentration as low as 1 ppm, but the responses were drastically different. In the case of the primitive MoS2 sensing channel, the resistance increased (positive response) upon exposure to all five representative VOCs (toluene, hexane, ethanol, propanal, and acetone) that were physically adsorbed on the surface. However, the thiolated MoS2 channel showed the opposite behavior for polar VOCs such as ethanol, propanal, and acetone; the resistance decreased by adsorption. The authors suggested that the formation of hydrogen bonding between the carboxylic acid group in the thiolated ligand and the oxygen in the analyte may promote charge transfer toward the MoS2 surface and decrease the resistance. The results indicate differences in the adsorption behavior based on the chemical nature of the analyte which can be utilized to distinguish the type (and the composition) of the VOCs. As the response of the MoS2 film can be easily tuned by ligand conjugation, the research team speculates that a versatile sensor array based on MoS2 can be constructed by conjugating a wide variety of thiolated ligands on the MoS2 surface and ultimately find its use in breath analysis devices for lung cancer diagnosis. The team is currently developing ligands for selective gas detection to enhance the sensing performance.