As a research project of the Global Frontier Program – KAIST Advanced Battery Center, Professor Seokwoo Jeon’s research team is working on the development of efficient electrocatalysts for water splitting reactions, particularly the oxygen evolution reaction (OER). The possibility of using layered transition metal chalcogenides such as MoS2 or WS2 for HER has attracted much research attention in recent years, but overall performance is generally limited by the lack of active sites on the basal plane, with only the edge sites being active.
Herein, the research team demonstrated that solution-phase substitution of phosphorus (P) in Cu2WS4 flakes improved the OER performance significantly. This low temperature solution-phase process provides an alternative to the traditional high-temperature, vapor-phase routes to produce P-based electrocatalysts, potentially making production more economical and environmentally friendly. Through high-resolution TEM analysis and other characterization techniques, it was revealed that numerous defects and amorphous regions form upon application of the solution-phase P-substitution method; these defects and regions can be beneficial for OER. The results showed that the catalyst with optimized chemical composition (Cu2WS3.4P0.6) had a low onset potential of 1.59 V vs. RHE at 10 mA cm-2, which is remarkable because the reference sample (with no P-substitution) is almost completely catalytically inactive under the same conditions. The Tafel slope of Cu2WS3.4P0.6 was calculated and found to be 194 mV/dec, close to that of the benchmark RuO2 catalyst (151 mV/dec). The catalyst also showed excellent stability in an alkaline environment, with no degradation in current density observed over 24 hours of continuous electrolysis in 0.1 M KOH. Density functional theory (DFT) calculations were performed to further elucidate the mechanism of enhancement, and revealed that the representative molecule with 3:1 ratio of S:P showed the lowest OH- adsorption energy at -6.51 eV.
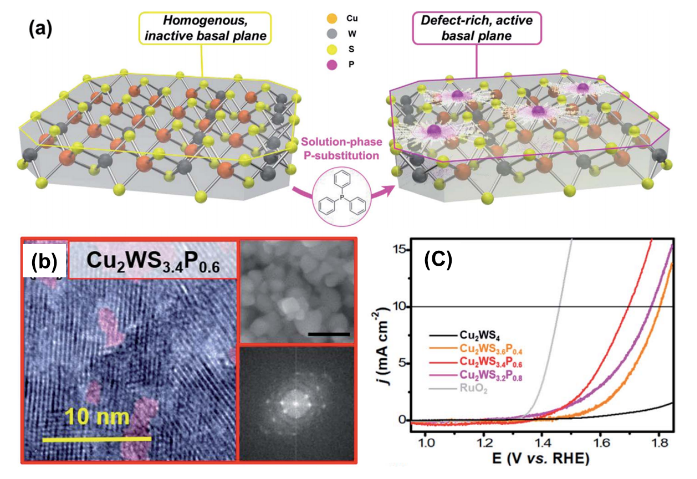
This work makes an important contribution to the development of efficient electrocatalysts for water splitting by outlining a new strategy for basal plane activation and P-substitution. We expect that this doping technique will be applicable to a wide range of other materials and eventually help to create low cost and efficient electrocatalysts.
Prof. Seokwoo Jeon, Mr. Travis G. Novak
Department of Materials Science and Engineering, KAIST (Advanced Battery Center, ABC)
Homepage: http://fdml.kaist.ac.kr
E-mail: jeon39@kaist.ac.kr, travisnovak@kaist.ac.kr






