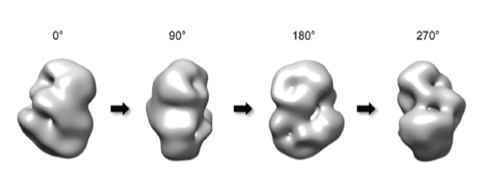
A group led by Professor Ji-Joon Song at KAIST Institute of BioCentury revealed the 3 dimensional structure of proteins that cause Huntington’s disease and identified the position of polyQ in the protein. While advances in medicine and medical treatments have substantially improved quality of life, neurodegeneration in the elderly have brought about serious medical and social problems. There are several neurodegenerative diseases including Alzheimer’s (AD), Parkinson’s (PD), Spinocerebellar Ataxia (SCA), and Huntington’s (HD) diseases. Among these diseases, HD and SCA belong to the polyglutamine (polyQ) tract disease family. The polyQ disease is a dominantly inherited neurodegenerative disorder that typically manifests itself during midlife with motor, psychiatric, and cognitive symptoms leading to death in 15-20 years after the onset. The polyQ disease is particularly interesting as it is caused by an expansion of the cytosine-adenine-guanine (CAG) repeat tract that encodes for polyQ in a single responsible gene, while multiple genes are responsible for AD and PD. This suggests that the polyQ diseases can be more responsive to therapy as a cure. Each polyQ disease is caused by polyQ expansion in different proteins, and differs in their pathologies. Therefore, the expansion is likely to alter the structure and function of the protein where the polyQ is embedded, which consequently leads to the diseases. Although each responsible gene for nine polyQ diseases has been known for decades, little is known about the effect of the polyQ expansion on the proteins in full-length context and there is no effective way to cure these diseases.
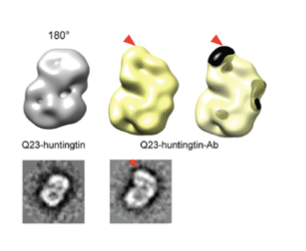
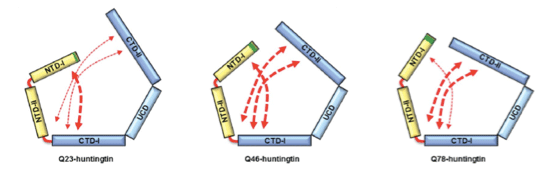
HD is caused by the polyQ expansion at the N-terminal in the huntingtin protein. Previous genetic studies have shown that huntingtin knockout plays an important role during the development of the protein. Despite the fact that huntingtin was identified to be responsible for HD two decades ago, little is known about the biological functions of the protein nor the molecular mechanism of the pathogenesis. According to the results from a structural study of huntingtin by the Song laboratory, the structure of the protein shows that Huntington’s disease protein posits a spherical shape that allows the N-terminal polyQ region to interact with the entire region of huntingtin. This suggests that the polyQ expansion affects the entire structure of the protein. Consistent with this structure, Song’s research has shown that the polyQ expansion, which is a primary cause of the disease, alters the entire structure of the protein as well as other biochemical properties. This work sets a structural framework to understand molecular basis of pathogenesis of the disease, and to develop a means to cure the disease.
Song, Ji-Joon (Associate Professor, Department of Biological Sciences)
Homepage: https://sites.google.com/site/songkaist/
E-mail: songj@kaist.ac.kr