In modern chemical industries, it is highly important to design a catalyst that can selectively convert a reactant into a desired product among various possible products. The groups of Prof. Minkee Choi and Prof. Hyungjun Kim at KAIST have proposed a new strategy to design chemoselective and long-lived metal catalysts by using unique interactions between metal catalysts and soft polymer supports. The study was published on July 8, 2020 in Science Advances (Dynamic metal-polymer interaction for the design of chemoselective and long-loved hydrogenation catalysts, Science Advances, eabb7369).
Metal catalysts, which play crucial roles in industrial hydroprocessing, have been conventionally supported on hard inorganic materials because of their high thermochemical stabilities. The use of soft matter, organic polymers, as a support for metal catalysts is relatively scarce because of their lack of thermochemical stabilities. However, when metal catalysts are supported on soft polymers, unique metal-support interactions and catalytic behaviors are expected to be achieved. For instance, mobile polymer chains can cover the entire surface of metal particles when a sufficiently strong metal-polymer interaction exists. The resultant 3-dimensional metal-polymer interface can strongly affect the transport and surface reaction of reactants, similar to the case of natural enzymes, which can be considered as metal catalysts embedded within biopolymers.
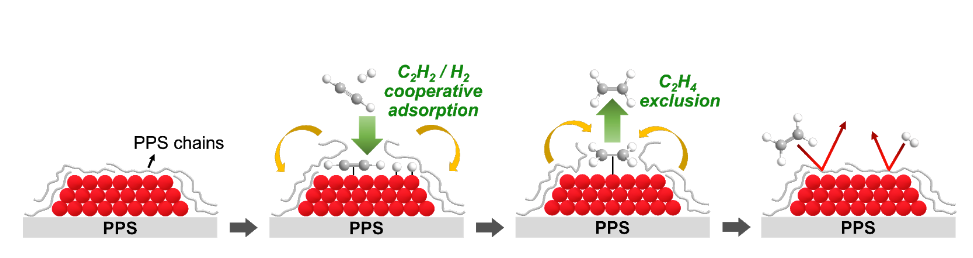
The researchers supported Pd catalysts on a thermochemically stable but “soft” engineering plastic, polyphenylene sulfide (PPS). They found that, near the glass transition temperature, the mobile PPS chains could completely cover the entire surface of Pd particles due to the strong interaction between Pd and PPS chains. Strikingly, they observed that the PPS chains decorating the Pd surface can act like a membrane and enable very selective adsorption/reaction of a target reactant. Using this phenomenon, they investigated the catalyst in an industrially relevant acetylene partial hydrogenation in the ethylene-rich stream. Ethylene, which is mainly produced by naphtha steam cracking in South Korea, is one of the most important chemicals in the petrochemical industry. Ethylene produced by naphtha cracking contains <1% acetylene impurity, which should be removed before the downstream ethylene polymerization process. Thus, it is important to selectively hydrogenate 1% of acetylene into ethylene, while keeping 99% of the ethylene chemically intact. The researchers found that the PPS overlayer covering the Pd catalyst surface enabled the exclusive adsorption and reaction of acetylene into ethylene (Scheme 1), while suppressing the adsorption and full hydrogenation of ethylene into ethane. The new catalyst also showed tremendously enhanced catalyst lifetime because the strong Pd-PPS interaction excluded the coke precursors from the Pd catalyst surface, thereby inhibiting detrimental coke deposition.
“We believe that designed polymers with diverse functionalities and molecular weights can be used to systematically tune the catalytic properties of supported metal catalysts,” Choi said. “Polymers do not need to be used alone and can be synergistically combined with inorganic materials to achieve optimal properties required from an engineering standpoint. Our ultimate goal is to realize ultrahigh selectivity of enzymes in industrial heterogeneous catalysts by mimicking nature.”
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea. This study was also partly supported by LG Chem.
Prof. Minkee Choi Dept. of Chemical and Biomolecular Engineering, KAIST
Prof. Hyungjun Kim Dept. of Chemistry, KAIST
Homepage: http://egcl.kaist.ac.kr
E-mail: mkchoi@kaist.ac.kr






