Luminescence from graphene-based quantum dots
Graphene is now a well-known two-dimensional material composed of a single atomic layer of carbon atoms. However many people do not know that graphene shines when the size of a graphene sheet becomes smaller than 100 nm. So-called graphene quantum dots (GQDs) are considered as a new type of quantum dot with excellent properties such as low cytotoxicity, excellent solubility, chemical inertia, wide absorption, stable photoluminescence, and better surface grafting abilities. Most of these properties cannot be easily achieved with conventional metal oxide-based quantum dots. Because of this, it is not surprising that GQDs hold promise in diverse applications including optoelectronic devices, sensors, bioimaging, and drug delivery.
It has been thought that the photoluminescent properties of GQDs are affected by their size, shape, and chemical structure. Particularly the existence of heteroatoms such as oxygen has been considered important as graphene is often derived from graphene oxide (GO), which can be obtained by oxidizing graphite to introduce oxygen and hydrogen. GO can be dispersed in a solution as single sheets and reduced to form graphene. Indeed, GQDs with different degrees of oxygen content (graphene oxide-quantum dots (GOQDs)) have been prepared by controlling the reduction process and their photoluminescent properties have been investigated. Another way should also be possible, i.e. producing GOQDs by exfoliation from oxidized graphite nanoparticles. However, GOQDs have yet to be produced with this method. And it is necessary to compare the GOQDs obtained by reduction (rGOQDs) to see if GOQDs with similar oxygen content show similar luminescent properties regardless of the preparation route.
This interesting question has been tackled by a collaborative research team in KAIST, organized by Professor Yong-Hoon Cho in the Department of Physics and Professor Tae Seok Seo in the Department of Chemical and Biomolecular Engineering. Their results not only show that the properties of GOQDs are actually route-dependent, but also provide important insights for understanding the optical properties of graphene-based QDs. The research was published in Small in August 2015 (http://onlinelibrary.wiley.com/doi/10.1002/smll.201500206/abstract) and were highlighted as a headlining article of the issue (http://onlinelibrary.wiley.com/doi/10.1002/smll.201570188/abstract).
Route-dependent luminescence from GOQDs
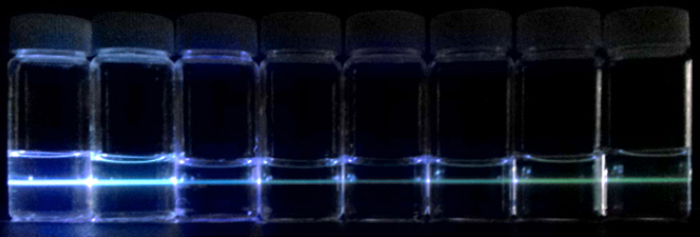
The team prepared GOQDs by exfoliation after oxidization of graphite nanoparticles using a mixture of sulfuric acid and nitric acid. In the GOQDs, the degree of oxygen content was controlled by the ratio of sulfuric acid and nitric acid. The GOQDs showed emission colors from sky-blue to greenish-yellow as the emission peak shifted as a function of the oxygen content, indicating the tunability of photoluminescence by controlling the oxidation reaction. However, the rGOQDs prepared by the reduction of fully oxidized GOQDs did not show a change in the peak position of emission spectra at 500 nm but only revealed the intensity at 440 nm which gradually increased as the reduction process proceeded further. The results clearly indicate that although GQDs and GOQDs can be reversibly synthesized via reduction and oxidation, GOQDs will exhibit different luminescent colors depending on the preparation route.
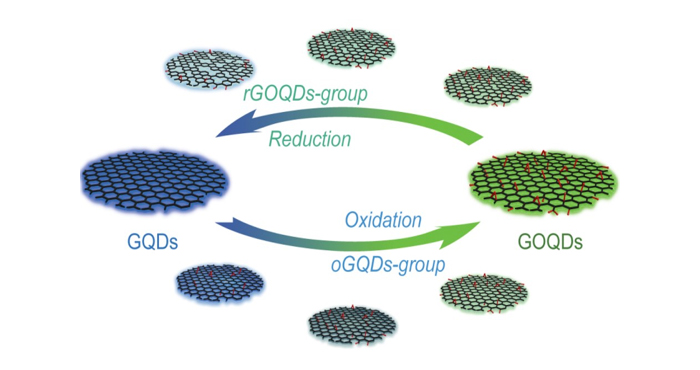
Through a combination of carrier dynamics and recombination study with first-principle density functional theory calculations, the authors revealed that the irreversible change in the photoluminescene from oxdiation to reduction stems from the different atomic structures. While the reduction process from fully-oxidized GOQDs produces isolated graphene nanodomains composed of pure carbon (sp2 bonding) surrounded by oxygen-containing domains (sp3 bonding), the oxidation reaction of the pristine graphene generates oxygen islands (sp3 bonding) on the graphene surface (sp2 bonding). As oxidation proceeds, the area of the sp3-bonding oxygen island will increase which results in a gradual change in the emission color. As the oxidative synthesis of GOQDs is more rapid than the reduction process and provides distinct advantages including tunable and stable photoluminescence, the team expects that this discovery will be useful for various applications such as bioimaging and functional nanoscale devices.
This work was mainly supported by the GRC project of KI for the NanoCentury and the National Research Foundation of the Ministry of Education.